Anatomy
 |
Fig. 1
(from: Manfra Marretta S. Dentistry and diseases of the oropharynx, in Birchard & Sherding (editors): Saunders Manual of Small Animal Practice, (3rd ed.). St. Louis. Elsevier, 2006, pp 632-635.) |
The
mandibular salivary gland is located at the bifurcation of the maxillary and
linguofacial veins. (Fig. 1) The duct empties at the sublingual papilla, which is
lateral to the most rostral aspect of the frenulum of the tongue. The gland has
a well-defined capsule. The sublingual gland has two distinct portions, the
monostomatic portion, which is immediately adjacent and rostral to the
mandibular gland, and the polystomatic portion, which are small islands of
gland dispersed along the salivary duct. The sublingual salivary duct is
closely associated with the mandibular duct. The parotid gland is located at
the base of the ear canal. Its duct empties adjacent to the upper 4th
premolar. This is a wide, but thin gland; not globoid like the mandibular
gland. The zygomatic gland is just ventral and medial to the zygomatic arch and
the duct empties 1 cm caudal to the parotid duct.
Etiology of mucocele
A
salivary mucocele is the accumulation of saliva outside of the gland or duct
system. The mucocele is not a true cyst since it does not have a secretory
lining. It can be due to a variety of causes. Trauma to the head or neck that causes
injury to either the gland or the duct can cause leakage of saliva. Oral
mucocele (ranula) is a reported complication of mandibulectomy in dogs and
cats. Infection or inflammation (sialadenitis) may cause enough tissue
disruption to allow leakage. Histopathology of salivary tissue removed to treat
mucoceles frequently reveals inflammatory changes. Although rare in dogs and
cats but more common in humans, calculi can cause obstruction of the salivary
duct with subsequent rupture of the duct and leakage. Rarely, neoplasia of the
gland can cause disruption of glandular or ductal tissues.
Mucoceles are classified by their
location. The cervical mucocele is the most common. A fluctuant mass is seen in
the submandibular region and the leaking gland or duct is either the mandibular
or the sublingual. If large, the mass may be mid-cervical or encompass the
entire ventral aspect of the neck. An oral mucocele, also called a ranula, is due to leakage from the
sublingual gland or duct and the swelling is intra-oral and lateral to the
tongue. A pharyngeal mucocele is very similar to cervical but the fluctuant
swelling is present in the pharynx, near the tonsil. Like the cervical
mucocele, the pharyngeal is due to leakage from either the mandibular or
sublingual salivary glands. Finally, the zygomatic mucocele can cause
exophthalmus and/or peri-orbital swelling and is due to leakage from the
zygomatic salivary gland.
Clinical Signs
Clinical signs of mucoceles vary
according to their location. The
cervical mucocele causes a fluctuant, non-painful swelling in the cranial
ventral cervical area. (Fig. 2)
 |
| Fig. 2: Cervical mucocele in a dog |
The mass usually lateralizes to the affected side. However,
in some cases the mass is directly on midline making it difficult to determine
whether the right or left salivary glands are causing the problem.
The oral mucocele, or rannula, can
cause dysphagia, anorexia, excessive salivation, abnormal movements or
protrusion of the tongue. (See previous post on Ollie for a case example.) The ranula may be large enough to be visible by the
owner. It appears cystic and can be large enough to deviate the tongue to the
opposite side.
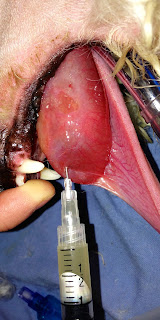
Dogs
with a pharyngeal mucocele (Fig. 3) can present for inspiratory stertor and dyspnea
since the fluid filled mass causes a physical obstruction in the pharynx and
upper airway.
 |
| Fig. 3: Pharyngeal mucocele (arrow) in a poodle |
Difficulty swallowing can also be a sign of pharyngeal mucocele.
A mucocele of the zygomatic
salivary gland can cause exophthalmus, divergent strabismus, and a fluctuant
swelling in the orbital area.
Diagnosis
In
most cases, salivary mucoceles are not a diagnostic challenge. The animals have
a history of an acute or chronic fluctuant swelling that is not painful.
Aspiration of the mass typically reveals a straw colored, mucinous fluid that
appears ropey when pushed through a needle and syringe onto a slide. (Fig. 4)
 |
| Fig. 4: FNA of a mucocele (www.acvs.org) |
Cytology
of the fluid reveals few cells unless the mucocele is very chronic or infected
in which case more evidence of inflammation is seen.
Diagnostic
imaging is usually not necessary but may be indicated in complicated cases or
ones in which the presentation is atypical. Ultrasound or computed tomography
may be helpful in animals with zygomatic mucoceles to differentiate the mass
from neoplasia or foreign body.
In
some animals, the cervical mucocele is difficult to lateralize because it is
either directly on midline or very large. Simple methods to determine the
affected side are to lay the dog on its back and see to which side the mucocele
gravitates. (Fig. 5)
 |
| Fig. 5: Placing the dog in dorsal recumbency can help lateralize the mucocele. |
Sometimes by pushing medially on the mucocele while doing an oral
exam the clinician can see one side of the pharyngeal wall bulge inward. In the
rare case where the affected side is still unclear after these manipulations,
positive contrast sialography can be performed. Contrast material is injected
into the salivary duct and the resultant images studied for evidence of leakage
or obstruction.
Treatment
Regardless of location, the definitive treatment of a
salivary mucocele involves removal of the offending salivary glands. Performing only drainage of the mucocele will not result in long-term resolution.
Surgical excision of mandibular and
sublingual glands is the treatment of choice for cervical mucoceles. (Fig. 6)
 |
Fig. 6: Excised mandibular (large gland to the left) and sublingual (remaining glands extending
from left to right) salvary glands |
The
mandibular gland, and the monostomatic and polystomatic portions of the
sublingual gland are removed as a unit since their ducts are closely
associated. The surgical approach is directly over the mandibular gland that
lies just cranial to the bifurcation of the jugular vein into the maxillary and
linguofacial veins. The capsule of the mandibular gland is incised to allow
dissection and removal of the gland. Dissection then proceeds cranially along
the mandibular duct. (Fig. 7)
 |
Fig. 7: Surgical excision of mandibular and sublingual salivary glands in a dog.
Retraction of the digastricus muscle (arrow) facilitates dissection. |
The multiple portions of sublingual gland are removed with
the mandibular gland and the ducts. Dissect the ducts as far cranially as
possible, then ligate and remove the tissues. Take care to avoid injury to the lingual nerve which lies over the salivary duct and serves as the most rostral limit of the gland and duct excision. Place a pen-rose or closed suction drain in the
mucocele and close the incision routinely.
The
etiology of pharyngeal mucoceles is similar to cervical mucoceles since they
are also caused by leakage from the mandibular or sublingual glands. Therefore,
remove these glands as described under cervical mucoceles. In addition, excise
the pharyngeal mucocele via an intra-oral approach. Make an elliptical incision
at the base of the mucocele, and excise the redundant mucosa and underlying
tissues to be sure that the interior of the mucocele has been exposed. Although
marsupialization of the resultant pharyngeal defect has been described, the
author simply leaves this incision open to heal by second intention.
An
oral mucocele, or ranula, is treated by “deroofing” the mucocele, followed by
marsupialization. (See previous post on Ollie.) Deroofing is performed by simply removing the mucosa over the
dorsal portion of the swelling being sure to expose the inside of the mucocele.
Marsupialization is performed by suturing the inner lining of the mucocele to
the oral mucosa. This allows the mucocele to remain open and drain into the
mouth. Suturing is usually done with an absorbable suture such as Monocryl or
PDS. Removal of the mandibular and sublingual salivary glands on the affected
side is also be performed to prevent recurrence.
The
zygomatic mucocele is treated by removal of the zygomatic salivary gland, which
is located just ventral to the eye and medial to the zygomatic arch. Removal of
the gland can be performed by partial removal of the zygomatic arch, or by
ventral orbitotomy. The zygomatic arch can also be temporarily removed to
provide exposure, and then reattached with orthopedic wire. Be careful not to
injure orbital structures during the dissection.
Postoperative Care
Submit
removed tissues for histopathology and culture. If a pen-rose drain was left in
the mucocele, remove it once drainage becomes minimal. If infection was
suspected or documented by positive cultures, treat the dog with appropriate
antibiotics for at least 7-10 days. After marsupialization of a ranula, feed
the dog soft food for 2-3 weeks, and flush the mouth with water or oral
antiseptic lavage after eating.
Complications
after treatment of a salivary mucocele are rare. A seroma can occur due to the
dead space created by the mucocele. Conservative treatment with warm compresses
is usually effective. Recurrence of mucocele is rare but may indicate
incomplete removal of the affected salivary tissue, or that the incorrect gland
was removed.
References
Bellenger CR, Simpson DJ. Canine sialocoeles – 60 clinical
cases. J of Sm. An. Practice
33:376-380, 1992.
Schmidt GM, Betts CW. Zygomatic salivary mucoceles in the
dog. J Am Vet Med Assoc 172:940-942,
1978.
Knecht CD. Diseases of the salivary glands in the dog. Comp Cont Ed II:932-938, 1980
Bartoe JT, Brightman AH, Davidson
HJ. Modified lateral orbitotomy for vision-sparing excision of a zygomatic mucocele
in a dog. Vet Ophthalmology10:127-131,
2007.
Manfra Marretta S. Dentistry and
diseases of the oropharynx, in Birchard & Sherding (editors): Saunders
Manual of Small Animal Practice, (3rd ed.). St. Louis. Elsevier,
2006, pp 632-635.
Benjamino K, Birchard SJ, Niles JD,
Penrod KD. Pharyngeal mucoceles in dogs: 14 cases. J Am Anim Hosp Assoc. 48(1):31-5, 2012

























.jpg)







