Permanent
tracheostomy is a well-recognized surgical technique used in animals and humans
as a salvage procedure to treat severe upper airway obstruction. Although the
technique has been used for many years with success, there are many
misconceptions among animal owners and veterinarians about the long-term care
and complications. Many feel that dogs cannot have a good quality of life
because of the problems associated with tracheostomy. Owners frequently expect
that dogs with permanent tracheostomy will have an appliance, i.e. a metal or
plastic tube that resides with in the trachea and needs constant care.
Indications for
tracheostomy in dogs include: severe laryngeal obstruction due to laryngeal
paralysis, collapse, neoplasia, or trauma, pharyngeal neoplasia that obstructs
the larynx, and non-resectable proximal tracheal neoplasia.
Although cats
may also develop disorders causing severe upper airway obstruction, permanent
tracheostomy is associated with frequent, severe complications such as
excessive mucous production and stoma stricture.(1) As a result, tracheostomy
is rarely recommended in cats.
Preoperative Considerations
Dogs being considered
for tracheostomy should be thoroughly evaluated with particular emphasis on the
respiratory tract. A complete
history and physical examination followed by appropriate imaging such as
thoracic radiographs are important before performing general anesthesia and
surgery. Cervical radiographs and even tracheoscopy may be necessary to be
certain that the respiratory tract downstream from the larynx is normal. Also,
carefully examine the dogs’ ventral cervical area to determine suitability for
creating a tracheostomy stoma. Some dogs, such as brachycephalic breeds, have
very short necks with excessive skin that can cause problems with skin flaping
over the stoma causing obstruction.
Surgical Technique
The dog is
placed in ventral recumbency with the neck hyperextended over a soft towel and
the front legs extended caudally. The ventral cervical area is clipped and
prepared for aseptic surgery. A ventral midline skin incision is made from the
larynx to just cranial to the manubrium. The paired sternohyoideus muscles are divided on their
midline using sharp dissection. A large horizontal mattress suture of 2-0 or
3-0 PDS is placed across the sternohyoideus muscles, dorsal to the trachea, to
allow retraction of the muscles and cause ventral displacement of the trachea.(Fig. 1)
Care is taken to avoid trauma to the recurrent laryngeal nerves during
passage of the suture. A rectangular
window is created in the trachea from the 3rd to the 7th
ring (4 rings included in the tracheal opening).(Fig. 2)
 |
| Fig. 2: The rectangular window is being created in the tracheal wall. Note the endotracheal tube present in the tracheal lumen. |
The tracheal incisions
are begun by incising between rings 3 and 4, then between rings 7 and 8. Be careful not to puncture the cuff of the endotracheal tube when making the initial tracheal incisions. These
parallel incisions are then connected using scissors to complete the
rectangular shaped defect in the trachea.(Fig. 2)
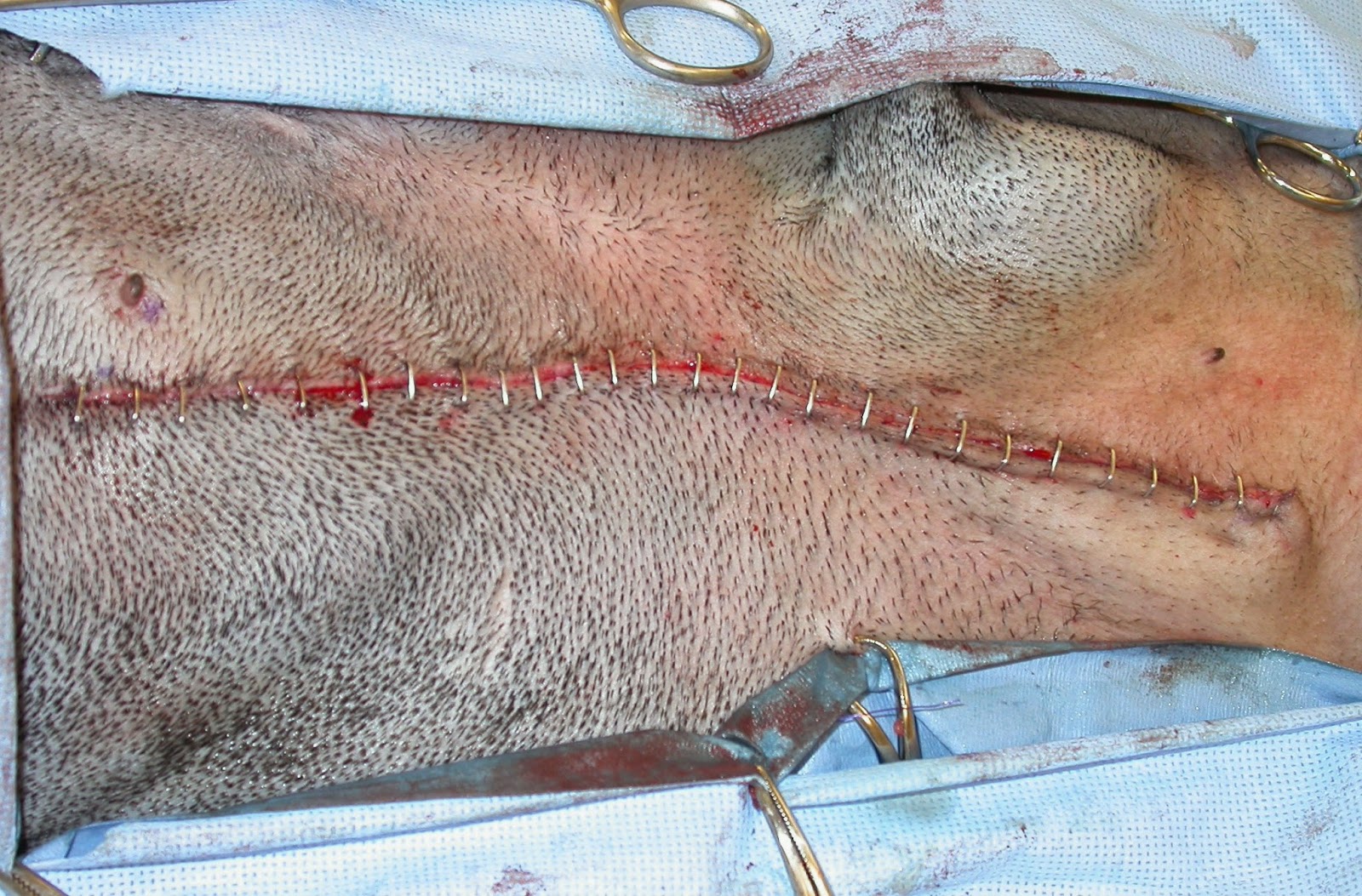
Close the
tracheal wall to the skin in a simple interrupted pattern to create the
tracheostomy stoma. Excise a rectangular shaped section of skin on each
side of the tracheostomy site to allow the skin incsion to match the
rectangular window in the trachea. The suture bites of trachea include the
cartilage, and the bites of the skin are placed split thickness, entering the
dermal layer and exiting the epidermis.
This allows for accurate apposition of the epidermis to the tracheal
mucosa. As in urethrostomy closure, take suture bites from inside out, i.e., start in the tracheal lumen and then take the bite of the skin. The corners of the window
are closed first (Fig. 3,4); then the remaining areas are closed in a similar
fashion.(Fig. 5) Absorbable suture such as 3-0 or 4-0 PDS is used to avoid having to remove them once the stoma has healed. The skin incisions cranial and caudal to the stoma are then
closed routinely.
 |
| Fig. 3: The 4 corners of the rectangular tracheal window are closed first. Note the "inside-out" sequence of suture placement. |
 |
| Fig. 4: The corner sutures have been placed. |
 |
| Fig. 6: Completed suture closure of the tracheal stoma. |
Postoperative Care
Alleviation of inspiratory dyspnea is immediate after permanent tracheostomy. See below video of an elderly labrador with laryngeal paralysis before and after permanent tracheostomy. Although laryngeal tie-back is the treatment of choice for most dogs with laryngeal paralysis, permanent tracheostomy was chosen in this dog due to high risk for aspiration pneumonia.
Besides routine postoperative care such as analgesics, cleansing of the stoma is important to prevent build up of discharge and debris. (Fig. 6) Gently wiping the skin around the stoma with moistened gauze sponges is sufficient.
 |
| Fig. 6: Typical appearance of a recently preformed permanent tracheostomy in a Yorkshire Terrier with severe laryngeal collapse. |
Owners should be advised to avoid putting anything inside
of the trachea and to not use any irritating materials around the stoma such as
peroxide or other antiseptics. Small amounts of a petroleum-based ointment
(e.g., triple antibiotic ointment) can be placed on the skin around the stoma
to prevent discharge from adhering to the skin and make cleaning easier. Discharge
from the tracheal stoma tends to gradually decrease over the first few weeks
postoperatively. Systemic antibiotics are not routinely prescribed since
incisional infections are very rare.
Life Style Limitations
Dogs with a
permanent tracheostomy cannot go swimming and should avoid very dusty
environments or running in tall grass or weeds. These dogs will also will have
difficulty barking or at least have a softer sound than pre-operatively. In
rare cases dogs with long hair will need clipping of the hair around the stoma
to prevent irritation of the tracheal mucosa and accumulation of debris.
Prognosis
Most dogs with permanent
tracheostomy do well and have minimal chronic problems. The most common
long-term postoperative problems are pneumonia and stricture of the stoma
requiring surgical revision.(2) In a recent study sudden death occurred after
tracheostomy in 5 of 19 dogs at variable times after surgery, presumably due to
obstruction of the trachea although necropsy was not performed in any of the
cases.(2)
Permanent
tracheostomy is considered an appropriate surgical option for dogs with severe
upper airway obstruction. Complications can occur but some, like stoma
stricture and skin fold occlusion, can be treated by revision surgery. Owner education
is important to explain potential risks and life style limitations.
References
1. Stepnik
MW1, Mehl
ML, Hardie
EM et. al. Outcome
of permanent tracheostomy for treatment of upper airway obstruction in cats: 21
cases (1990-2007). J Am Vet Med Assoc. 2009 Mar
1;234(5):638-43.
2. Lindsay
L. Occhipinti and Joe G. Hauptman. Long-term
outcome of permanent tracheostomies in dogs: 21 cases (2000–2012) Can Vet J. Apr 2014; 55(4): 357–360.